The Applied Computational Psychiatry lab focuses on developing computational tools with real potential for clinical applications.
Computational Psychiatry is a multidisciplinary field of research at the intersection of psychiatry, neuroscience, machine learning and statistics. The aim of the field is to harness advances in these fields to advance treatments for mental illnesses. For overviews over computational psychiatry, see Huys et al., 2016 Nature Neuroscience and Huys et al. 2020 Neuropsychopharmacology
We are part of the Divison of Psychiatry and the Max Planck UCL Centre for Computational Psychiatry and Ageing Research in the Institute of Neurology at University College London.
When an organ is unable to meet the demands placed on it, illness can arise. As the main functions of the brain are to compute and learn, an understanding of mental illnesses will benefit from an understanding of the computational and learning functions the brain performs, and how these are affected in states of ill-health.
Latest Publications
Hidden state inference requires abstract contextual representations in the ventral hippocampus.
Mishchanchuk, Karyna; Gregoriou, Gabrielle; Qü, Albert; Kastler, Alizée; Huys, Quentin J. M.; Wilbrecht, Linda; MacAskill, Andrew F. (2024).
Science, vol. 386, iss. 6724, pp. 926–932

The ability to use subjective, latent contextual representations to influence decision-making is crucial for everyday life. The hippocampus is hypothesized to bind together otherwise abstract combinations of stimuli to represent such latent contexts, to support the process of hidden state inference. Yet evidence for a role of the hippocampus in hidden state inference remains limited. We found that the ventral hippocampus is required for mice to perform hidden state inference during a two-armed bandit task. Hippocampal neurons differentiate the two abstract contexts required for this strategy in a manner similar to the differentiation of spatial locations, and their activity is essential for appropriate dopamine dynamics. These findings offer insight into how latent contextual information is used to optimize decisions, and they emphasize a key role for the hippocampus in hidden state inference.
Amygdala Reactivity, Antidepressant Discontinuation, and Relapse
Erdmann, Tore; Berwian, Isabel M.; Stephan, Klaas Enno; Seifritz, Erich; Walter, Henrik; Huys, Quentin J. M. (2024).
JAMA Psychiatry, vol. 81, iss. 11, pp. e242136
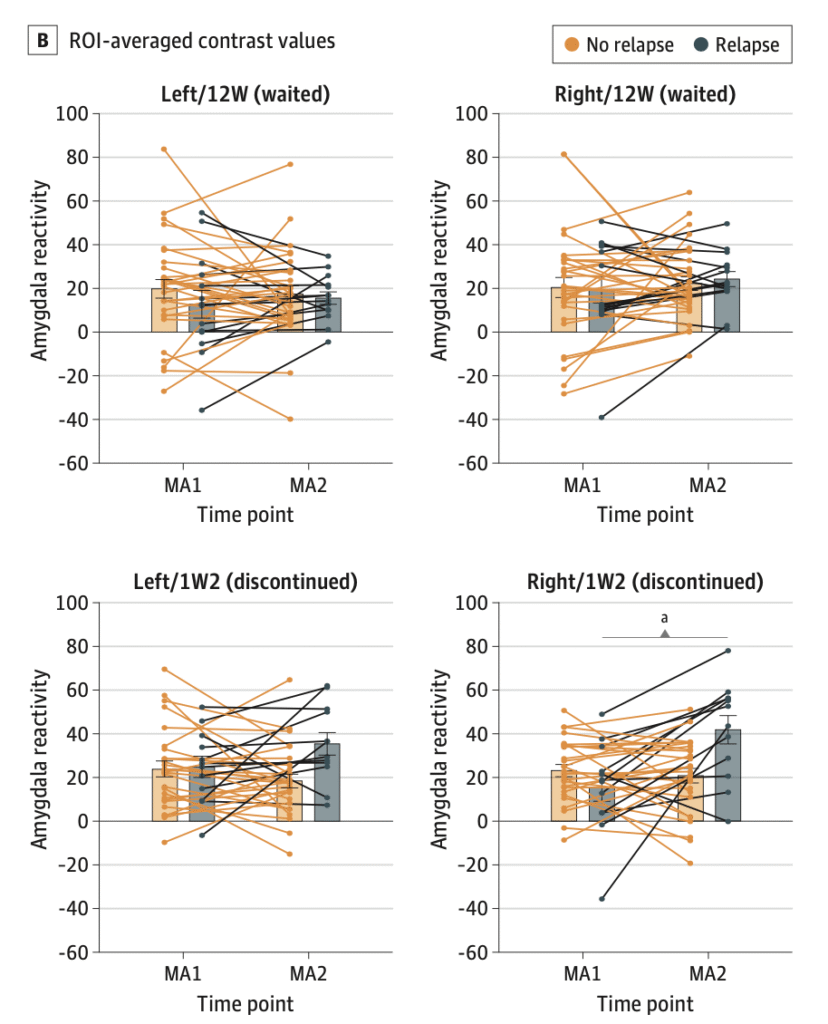
Antidepressant discontinuation increases depression relapse risk. The AIDA study investigated amygdala reactivity changes after discontinuation and their relation to relapse risk. This fMRI study included 80 remitted MDD patients and 53 controls. Amygdala reactivity was measured before or after discontinuation, with relapse monitored for 6 months. Increased reactivity after discontinuation was associated with depression relapse (β = 18.9, P = .04), predicted shorter time to relapse (HR = 1.05, P = .01), and relapse occurrence (67% accuracy, P = .02). These findings suggest amygdala reactivity changes may indicate relapse risk in remitted MDD patients discontinuing antidepressants.
Latest Preprints
Day-to-day fluctuations in motivation drive effort-based decision-making.
Hewitt, Samuel RC; Norbury, Agnes; Huys, Quentin J. M. and Hauser, Tobias U. (2025).
PANS, vol. 122, iss. 12, pp. e2417964122
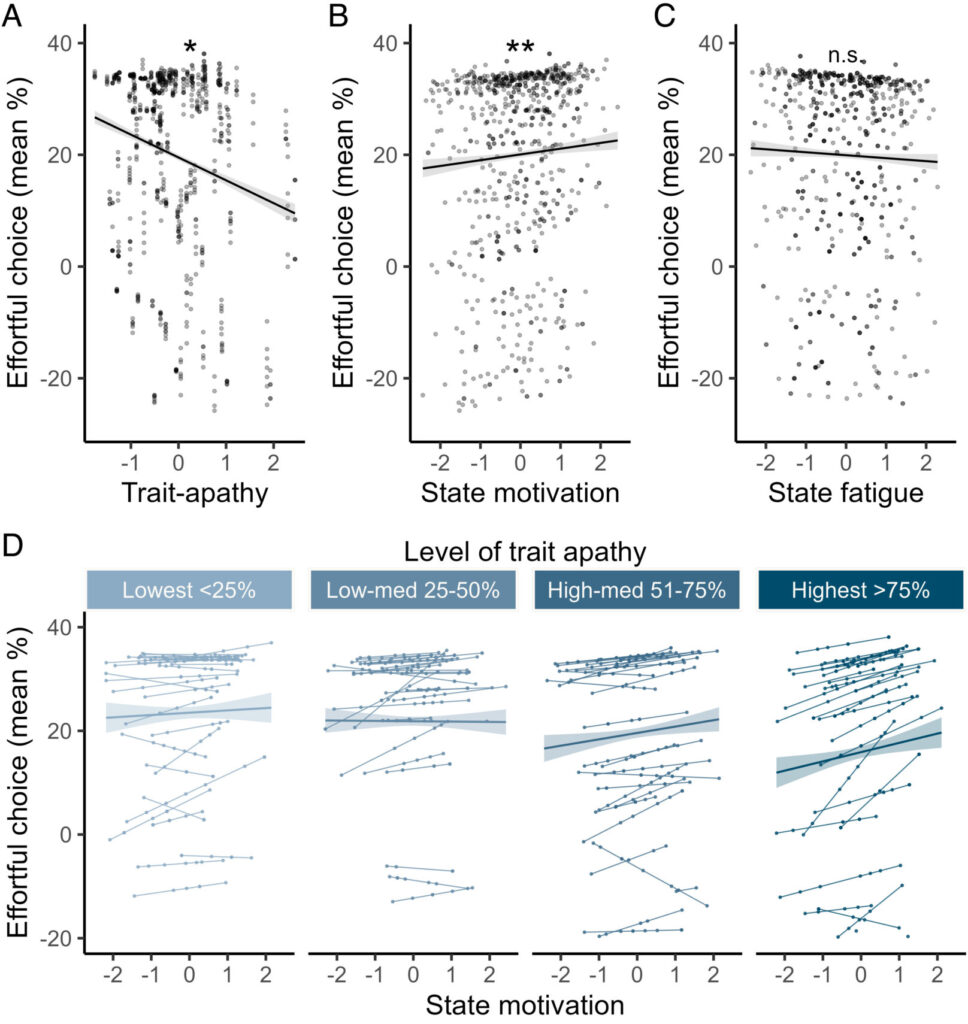
Subjective experiences, like feeling motivated, fluctuate over time. However, we usually ignore these fluctuations when studying how feelings predict behaviour. Here, we examine whether naturalistic ups and downs in states influence the subjective value of choices.
Task-based willingness to exert effort for reward was specifically boosted when people felt more motivated. This naturalistic state-behaviour coupling was significantly strengthened in individuals with higher trait apathy. Computational modelling revealed that the fluctuations in state changed and preceded sensitivity to reward, thereby driving choices.
Our results show that typical, day-to-day fluctuations in feelings and cognition are tightly linked, and critical to understanding fundamental human behaviours in the real-world.
Mkrtchian, Anahit; Qiu, Zeguo; Abir, Yaniv; Erdmann, Tore; Dercon, Quentin; Sedlinska, Terezie; Browning, Michael; Costello, Harry; Huys, Quentin J. M. (2025).
JAMA Psychiatry, doi:10.1001/jamapsychiatry.2025.0839

Question: Do pharmacological manipulations of dopamine and serotonin affect components of reinforcement learning in humans?
Findings: Upregulating dopamine increases reward learning/sensitivity and reward response vigor, and decreases reward discounting. Upregulation of serotonin leads to increased punish- ment learning/sensitivity and decreased reward discounting.
Meaning: Pharmacological manipulations of dopamine and serotonin have dissociable effects on different components of reinforcement learning. This forms a necessary basis for the devel- opment of selective markers for treatment assignment.